CORROSION – AN OVERVIEW
Corrosion is a natural phenomenon which can be said as degradation of material through a chemical process which is environmentally induced, resulting in material and economic losses to the society impacting infrastructure of Industries, Public utilities and other structures. The material loss will require maintenance and replacement which involve costs and burden to the economy
Corrosion is a process where many factors play to drive the process of corrosion, like varying environment (humidity), temperature, pollution, air current, metal distribution, orientation etc
IMPACTS OF CORROSION
There are many aspects/areas where corrosion impacts like
|
Aspect |
Examples |
|
Health and safety |
Containers becoming weak leading to leakage and collapse, Structures becoming weak etc, corrosion products caused by body implants like pins, plates, joints etc |
|
Environment |
The leeching of corrosion products into the environment cause both land and water pollution |
|
National treasures and artefacts |
Highly polluted environments accelerate corrosion of artefacts leading to serious deterioration of artistically and culturally significant items |
|
Fuel Infrastructure |
Pipelines getting corroded leading to spillage and corrosion due to biofuels etc, Home appliances, pipelines, Industrial equipment breakdowns, supports, vessels, structures are very few examples that are impacted by corrosion. |
|
Public infrastructure |
Bridges collapsing due to corrosion, highway infrastructure, ships getting corroded |
|
Industrial infrastructure |
Industrial equipment breakdowns, supports, vessels, structures are very few examples that are impacted by corrosion. |
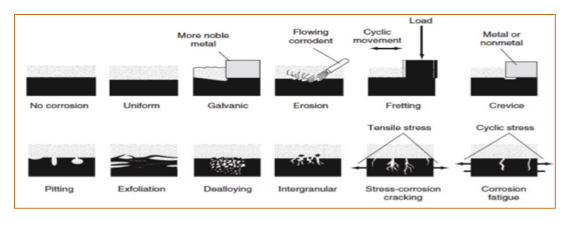
TYPES OF CORROSION
Corrosion is categorized into Metallic corrosion and Non-Metallic corrosion
METALLIC CORROSION: These are generally classified into
- Uniform corrosion
- Galvanic corrosion
- Erosion corrosion
- Fretting corrosion
- Crevice corrosion
- Pitting corrosion
- Exfoliation Corrosion
- Dealloying Corrosion
- Intergranular Corrosion
- Stress- cracking corrosion
- Corrosion fatigue

Galvanic Corrosion: This type of corrosion is due to the difference in electrical potentials of two metals in contact with each other. Due to this difference in electric potential, there is an electrical path created between two metals in a conducting environment.

Erosion – Corrosion: This type of corrosion id due to the deterioration of metals and alloys to the relative movement between the corrosive fluid and the metal surface, like impellers and tubes etc

FRETTING CORROSION: Fretting corrosion is a type of deterioration at the interface between contacting surfaces. It is severe wear that occurs to heavily loaded surfaces that move against each other only slightly but repeatedly. Fretting corrosion is different occurs rapidly with little movement which differentiates with ordinary wear

CREVICE CORROSION: Crevice corrosion is a localized form of corrosion usually associated with a stagnant solution in the crevices, like gaskets, washers, fastener heads, lap joints and clamps. This attack occurs due to the part of the metal surface is in a shielded environment, compared to the rest of the metal which is exposed to the electrolyte

PITTING CORROSION: Pitting corrosion is a localized form of corrosion by which cavities or "holes" are produced in the material. It is considered more dangerous than uniform corrosion damage
EXFOLIATION CORROSION: Exfoliation corrosion is a form of localized corrosion that occurs when corrosion propagates along intergranular paths parallel to the material surface. This Corrosion product creates stress at the grain boundaries Separate layers of metal are formed due to the wedging action resulting in damage first to the metal surface then extending into the interior as the corrosion propagates. This form of corrosion is associated with high strength aluminium alloys

DEALLOYING CORROSION: Dealloying or selective leaching refers to the selective removal of one element from an alloy by corrosion processes. It is also called selective leaching. An example is the selective removal of zinc from a brass alloy
INTERGRANULAR CORROSION: It is a localized attack along the microstructural grain boundaries of metals and alloys. This form of corrosion is due to the presence of impurities

CORROSION FATIGUE: This form of corrosion is due to the combined effect of alternating stresses and corrosive environment. The fatigue process will weaken and rupture the protective layer which makes the corrosion faster.
MITIGATION MEASURES TO PREVENT CORROSION
Even though corrosion is a natural process, still it can be prevented or slowed down. The main ways through which corrosion can be substantially prevented are
- Protection through the application of paints/coatings
- Material selection and design
- Cathodic protection
- Anodic protection
- Inhibitors
- There are different corrosion tables available for various materials which can be chosen depending upon the corrosive environment like stainless steel for handling nitric acid, Monel metal alloys for hydrofluoric acid, Nickel alloys for storing caustic etc.
- Addition of chromium in steel will passivate the material, Addition of arsenic and antimony in brass acts as an inhibiting agent, desensitization of stainless-steel welds will prevent corrosion by bringing changes to the microstructure. Introduction of surface compressive stress will improve corrosion fatigue and stress corrosion cracking
- Ensure no retention of water while complete draining, avoiding contact with dissimilar metals, wherever possible welding to be done than riveting
- To protect marine structures which are highly subjected to corrosion. The structure can be made more cathodic by either using sacrificial nodes or through impressed currents and also by making passivating metal structures more anodic.
- Metallic Coatings help in the prevention of corrosion by acting like a protective layer, there are many methods of coatings used like galvanizing (thin coating of zinc on the iron), Electroplating (Coating of copper, nickel or chromium with use of direct current), tin plating (Coating of tin on the iron), cladding etc.,
- Organic coatings like paints, enamels and varnishes are used to protect metallic surfaces. Inorganic coatings are produced by chemical or electrochemical reactions at the metal surface to protect the base metal from corrosion.
Article by PJ Mohan
Sr.Faculty, NIFS




No comments:
Post a Comment